- Midbrain dopaminergic neuron development
- Characterizing the molecular cascades that regulate midbrain stem cell behavior as well as dopaminergic neurogenesis, differentiation and subtype specification.
- The functions of Wnts, their receptors and their signaling pathways, with focus on new components of the Wnt/PCP signaling.
- The function of nuclear receptors and their ligands in midbrain dopaminergic neuron development.
- The identification of novel factors by single cell transcriptomic, proteomic and lipidomic approaches.
- Control of midbrain dopaminergic neurogenesis by basic-helix-loop-helix transcription factors.
- Stem cells and direct reprogramming for Parkinson’s disease (PD) cell replacement therapy and drug discovery.
- Improving protocols for the efficient differentiation of human stem cells (NES, ES, and iPS cells) into substantia nigra A9 dopaminergic neurons for cell replacement therapy and drug discovery.
- Develop 2D and 3D in vitro models of midbrain tissue for PD drug discovery using human NES/ES/iPS cells with PD mutations and isogenic controls.
- Examine the role of Wnt signaling in the pathogenesis of PD.
- Improve protocols for the direct in vitro reprogramming of somatic cells into substantia nigra A9 dopaminergic neurons.
- Develop a novel cell replacement therapy for PD based on the direct in vivo reprogramming of striatal astrocytes in situ, into functional A9 dopaminergic neurons.
Background
Parkinson's disease (PD) is one of the most common neurodegenerative diseases in the world, affecting about 2% of the population over 65. This progressive disease is often associated with the symptoms of resting tremor, rigidity, bradykinesia, and postural instability. These symptoms are attribured to the loss of a specific cell type in the substantia nigra pars compacta part of the midbrain. These dopaminergic (DA) neurons project to other regions of the brain including the striatum that controls such things as fine motor control. The pathological features include protein aggregates in DA neurons composed of alpha-synuclein referred to as Lewy body inclusions. The underlying cause of PD, however, is still speculative with evidence reported ties to environmental factors and in less than 10% of cases some genetic link even though they are not fully penetrant.
Current treatments of PD do not address the underlying cause of the disease but rather focus on symptomatic relief. Treatments include the use of a precursor to dopamine L-DOPA, other dopamine agonists, inhibitors of dopamine degrading enzymes, and more invasive methods such as surgical implantation of electrodes for deep brain stimulation. These treatments have shown benefits but do not change the progressive course of PD and over time have reduced efficacy and side effects. New restorative and regenerative approaches are thus being developed as a therapeutic alternative (Summarized in the figure below). Cell replacement therapy has been shown to work in clinical trials in PD using fetal tissue and provided a proof of concept for future development into this therapy. In order to fully implement CRT into the clinic, a cell source that is easily accessible, up-scalable and standardized is needed. Over the years, many groups have investigated other possibilities for CRT and has lead to profound advancements in the field. The knowledge obtained from this understanding can then be applied to ongoing stem cell differentiation protocols, to improve the efficiency and outcome of DA cells that could one day be used for CRT in PD patients.
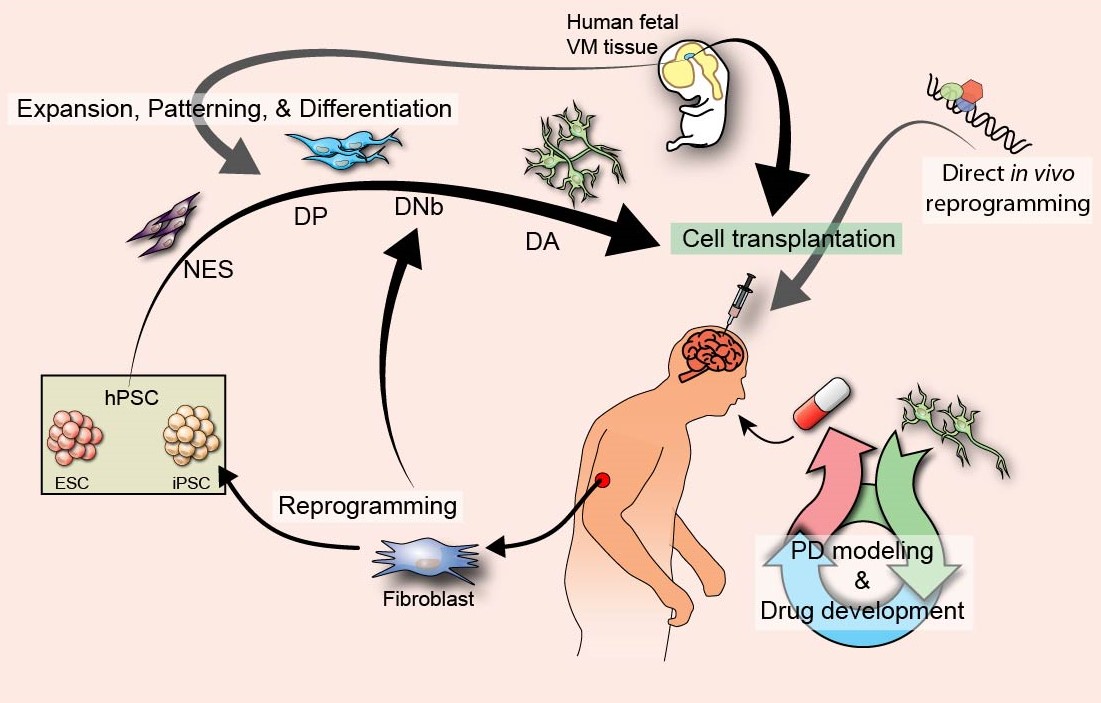
Summary of cell replacement strategies for Parkinson's disease.
Current alternate approaches for treatment of PD such as deriving DA neurons from various hPSC sources including reprogrammmed somatic cells.
One can also use reprogramming to directly transdifferentiate from one cell type to a DA cell type. Human fetal tissue has already been used
in clinical trials and shown proof of concept that CRT works. Other possible approaches include reprogramming in vivo, and using alternate strategies
such as high throughput drug screening in vitro. Abbreviations (hPSC: human pluripotent stem cell; ESC: embryonic stem cell; iPSC: induced
pluripotent stem cell; NES: neuroepithelial stem cells; DP: dopaminergic progenitor; DNb: dopaminergic neuroblast; DA: dopaminergic neuron)
Studying ventral midbrain development in vivo can give insight into complex molecular programs that could be used to recapitulate the process in vitro. This would aid in having differentiation protocols that specify for the correct cell types required for CRT. The current understanding of DA development is reviewd in Arenas et al. Development 2015. The future of ventral midbrain DA development research could provide the key to translational research to the clinic. Beyond classical developmental approaches, ongoing research inlcudes looking into subtle differences between mouse and human development, as well as looking how extrinsic factors influence development in the form of extracellular components and their role being applied to differentiation protocols.
Postdoctoral research positions
Please, send your inquiries by e-mail to ernest.arenas@ki.se and include the following information:
- CV with publications and research experience
- A brief outline of research interests
Top picture panel (left to right): Differentiated hESCs, LMX1A/FOXA2 ICC (K. Nishimura); Mouse in situ, Th (Allen Brain Atlas); Bright field differentiated hESCs (K. Nishimura); Mouse VM, TH/NR4A2 IHC (D. Gyllborg); Bioinformatics analysis; Differentiated hESCs, TH/MAP2 ICC (K. Nishimura).